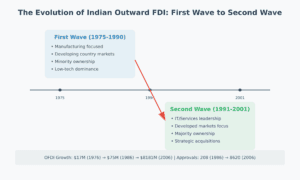
PART 1: SETTING THE STAGE
The Big Challenge Small and medium pharmaceutical companies in India face a critical moment: adapt to global competition or risk being left behind. While they’ve historically been the backbone of India’s pharmaceutical industry – making up 91% of companies and employing 65% of the workforce – their share of exports has plummeted from 37% in 1986 to just 2.4% in 2001. What’s holding them back, and more importantly, how can they break into global markets?
Why This Matters
- For the Economy: SMEs are crucial for employment, regional development, and affordable medicine
- For Innovation: They’re often more nimble and can fill market niches
- For Global Health: They can provide affordable medicines worldwide
A Tale of Two Worlds: Large vs Small Pharma Imagine two pharmaceutical companies in India:
- Large Corp: Well-funded R&D, international offices, sophisticated marketing
- Small Corp: Limited resources but nimble, struggling with basic modernization
The gap is widening. Large Indian pharma companies are going global while SMEs struggle to keep up. But some small companies are breaking through – how are they doing it?
PART 2: THE SUCCESS FORMULA
The Four Pillars of Global Success Based on extensive research, successful pharmaceutical SMEs share key characteristics:
- Innovation Matters Most
- Case Study: Tonira Pharma
- Started small but invested 10% of sales in R&D
- Now exports to 40 countries
- Key lesson: Even small R&D investments can yield big returns
- Quality is Non-Negotiable
- The GMP Challenge
- Good Manufacturing Practices are essential for exports
- Success story: How Ozone Pharmaceuticals became an early GMP adopter
- Practical guide: Steps to achieve GMP certification
- Strategic Marketing
- Beyond Price Competition
- Building international networks
- Finding niche markets
- Using digital marketing effectively
- Smart Financing
- Making Limited Resources Work
- Government schemes available
- Creative financing options
- Cost-effective expansion strategies
PART 3: PATHWAYS TO GOING GLOBAL
The Export Route: First Steps Abroad Think of entering global markets like learning to swim – most companies start in the shallow end (exports) before diving into deeper waters (foreign investments).
Success Stories That Light The Way
- The Innovation Champion: Fermenta Biotech
- Before: Small enzyme manufacturer
- Strategy: Heavy R&D investment (9% of sales)
- Result: 41% export intensity
- Key Lesson: Found their niche in specialized enzymes
- The Quality Leader: N G L Fine-Chem
- Focus: Veterinary medicines
- Strategy: ISO certification + cGMP compliance
- Result: 70% export intensity
- Key Lesson: Quality standards opened global doors
- The Market Pioneer: Paras Pharmaceuticals
- Approach: Created subsidiaries in UAE and USA
- Strategy: Used foreign offices as launch pads
- Result: Doubled exports in two years
- Key Lesson: Local presence matters
The Investment Route: Taking The Plunge
Five Ways to Invest Abroad (From Lowest to Highest Risk)
- Liaison Offices
- Like having a business card in foreign markets
- Low cost, low risk
- Examples: Jagsonpal’s offices in New York, Belarus
- Marketing Joint Ventures
- Sharing costs and risks with local partners
- Access to established networks
- Case Study: Venus Remedies’ European strategy
- Sales Subsidiaries
- Full control over marketing
- Higher investment needed
- Success Story: Paras Inc. in USA
- Manufacturing Joint Ventures
- Access to local production
- Technology sharing risks
- Real Example: Rusan Pharma’s approach
- Manufacturing Subsidiaries
- Complete control
- Highest risk and investment
- Case Study: Venus Pharma GmbH in Germany

PART 4: THE GOVERNMENT’S TOOLBOX
Current Support Systems
- Financial Support
- Special venture capital funds
- Priority sector lending
- Tax benefits for R&D
- Technical Support
- Quality certification help
- Training programs
- Research collaboration support
- Market Support
- Export promotion councils
- Trade fair assistance
- Market intelligence
What’s Missing: The Reform Agenda
- Information Gap
- Current: Many SMEs unaware of available support
- Needed: Better communication and outreach
- Solution: Local information centers and online portals
- Financial Support
- Current: Complex procedures, high collateral requirements
- Needed: Simpler, more accessible funding
- Solution: Special SME export finance schemes
- Technical Support
- Current: Limited access to testing facilities
- Needed: Shared infrastructure
- Solution: Cluster-based common facility centers
- Market Intelligence
- Current: Fragmented information
- Needed: Comprehensive market data
- Solution: Centralized export intelligence system
PART 5: PRACTICAL ROADMAP FOR SMEs
Step-by-Step Guide to Going Global
- Assessment Phase (3-6 months)
- Evaluate readiness
- Identify target markets
- Review resource requirements
- Preparation Phase (6-12 months)
- Upgrade quality systems
- Develop export strategy
- Build necessary capabilities
- Market Entry Phase (12-18 months)
- Start with easier markets
- Build relationships
- Learn from experience
- Expansion Phase (18+ months)
- Increase market presence
- Consider foreign investments
- Build global networks
Critical Success Factors
- Start with strong domestic base
- Focus on quality from day one
- Invest in R&D within means
- Build international networks
- Use government support effectively
Common Pitfalls to Avoid
- Rushing into difficult markets
- Neglecting quality standards
- Underestimating resource needs
- Ignoring market research
- Going it alone without support
The Bottom Line Indian pharmaceutical SMEs can succeed globally with the right strategy, support, and persistence. While the challenges are real, the opportunities are greater. Success requires:
- Strategic approach to internationalization
- Focus on building core capabilities
- Effective use of government support
- Learning from successful examples
The future belongs to those who can combine the agility of small firms with global standards and reach. It’s a challenging journey, but as the success stories show, it’s entirely possible with the right approach and support.
Academic Abstract:
In a rapidly globalizing world, India’s small and medium pharmaceutical enterprises (SMEs) face a critical turning point. Once comfortable in a protected domestic market, these companies must now compete globally or risk obsolescence. This transformation isn’t just about corporate survival—it’s about maintaining India’s pharmaceutical ecosystem, which relies on SMEs for employment, drug production, and affordable healthcare.
This book presents the first comprehensive analysis of how Indian pharmaceutical SMEs can successfully enter global markets through exports and foreign direct investment (OFDI). Through rigorous empirical analysis and in-depth case studies, it reveals both the obstacles and opportunities these companies face. The research breaks new ground by providing the first detailed sizing of India’s pharmaceutical SME sector and employing advanced methodological approaches to analyze their export behavior.
The findings challenge conventional wisdom about small companies’ limitations. While pharmaceutical SMEs show significant potential for international expansion, they face a triple constraint: limited financial resources, technological capabilities, and policy support. The book demonstrates how some SMEs have successfully overcome these barriers through innovation, strategic partnerships, and efficient use of available resources.
Based on these insights, the study proposes a comprehensive policy framework to support SME internationalization. Key recommendations include providing accessible low-cost financing, strengthening connections with research institutions, implementing SME-specific incentive structures, developing pharmaceutical clusters, and creating targeted training programs for global market entry.
This research offers valuable insights for policymakers, business leaders, and researchers interested in understanding and supporting the internationalization of small and medium enterprises in knowledge-intensive industries.
Learn More:
Full citation: Pradhan, Jaya Prakash and P.P. Sahu (2008), Transnationalization of Indian Pharmaceutical SMEs, New Delhi: Bookwell Publisher.
Learn More: