Imagine walking into a pharmacy in India in the 1970s versus today. Back then, most medicines were imported and expensive. Now, India is known as the “pharmacy of the world,” producing life-saving drugs at a fraction of global prices. This remarkable transformation didn’t happen by chance – it’s the result of careful policy choices that are now facing unprecedented changes.
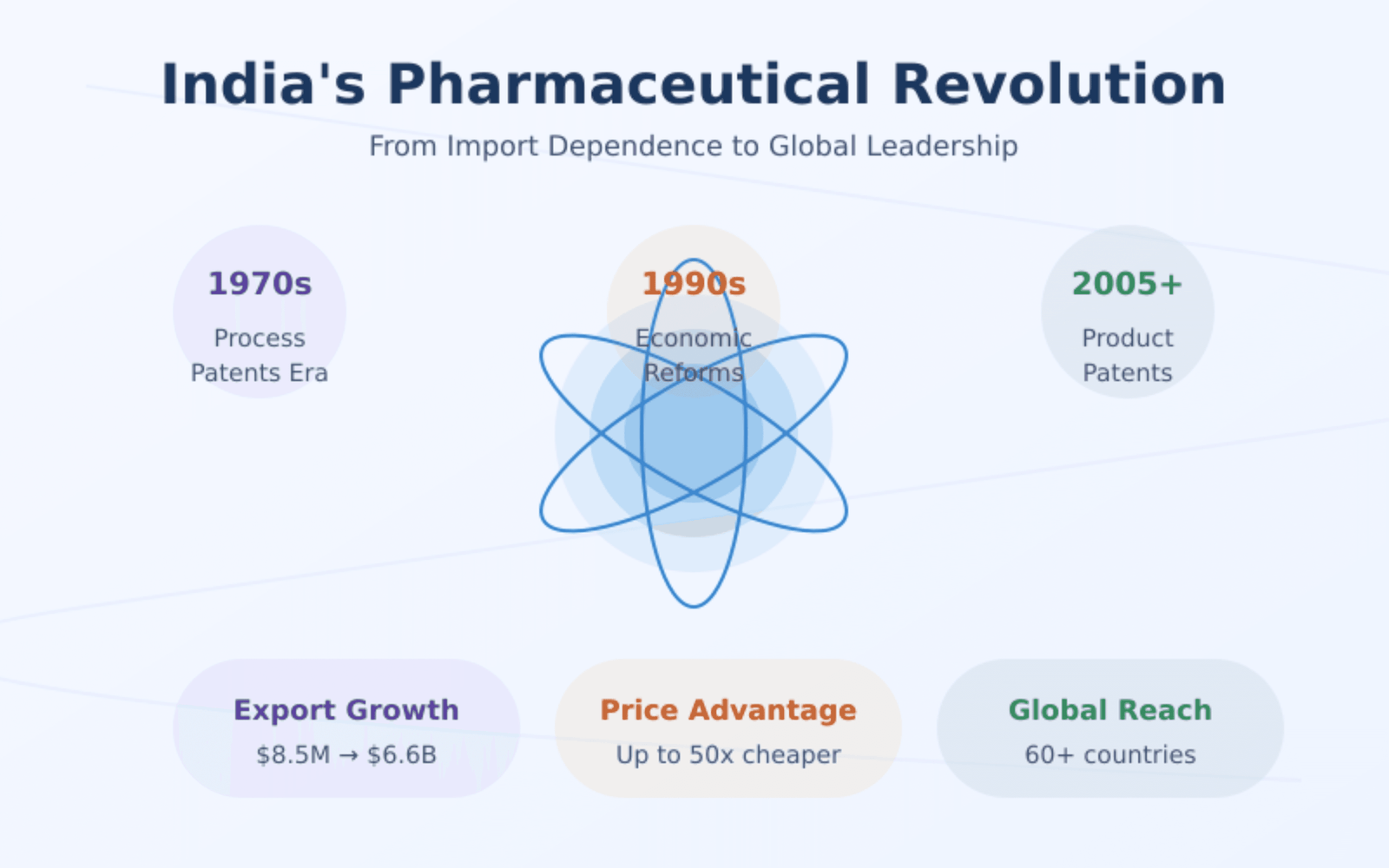
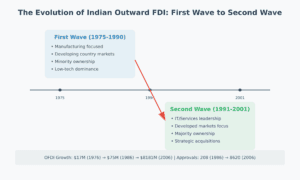
The Rise of India’s Pharmaceutical Revolution Think of India’s pharmaceutical industry as a carefully tended garden that grew from modest seedlings to a flourishing ecosystem. In the early days after independence, the country was heavily dependent on imported medicines. But through strategic policies, including:
- A patent system that protected processes rather than products
- Price controls to keep medicines affordable
- Incentives for domestic manufacturing
- Support for research and development
This nurturing environment helped create a robust industry that today not only meets domestic needs but exports to over 60 countries.
The Secret Sauce: Process Innovation Indian companies became masters at finding new, cost-effective ways to make existing medicines. Think of it like discovering a more efficient recipe for baking bread – the end product is the same, but the process is better and cheaper. This expertise allowed Indian companies to produce medicines at prices sometimes 50 times lower than in Western markets.
The Global Impact The success story isn’t just about business growth. Indian pharmaceutical companies have played a crucial role in global health by:
- Making HIV/AIDS treatments affordable in Africa
- Providing essential medicines to developing countries
- Becoming a reliable supplier of quality generic drugs worldwide
The New Challenges But now this garden faces climate change, so to speak. Major policy shifts are transforming the landscape:
- WTO requirements for stronger patent protection
- Reduced price controls
- More open competition from global companies
Studies suggest these changes could increase drug prices significantly – some estimates range from 26% to 242% – potentially putting essential medicines out of reach for many.
The Way Forward For Indian pharmaceutical companies, the future requires adapting to this new reality through:
- Increased investment in research and development
- Focus on niche areas like traditional medicine
- Strategic partnerships with global companies
- Protection of domestic industry leaders
- Careful use of WTO flexibilities to maintain access to essential medicines
A Global Health Pioneer at a Crossroads India’s pharmaceutical industry isn’t just a business success story – it’s a model for how developing countries can build industries that serve both economic and social goals. As one researcher noted, “India proved that providing affordable medicines and building a world-class industry aren’t mutually exclusive.”
The challenge now is maintaining this delicate balance in a changing global environment. The decisions made today will affect not just India’s pharmaceutical industry, but global access to affordable medicines for years to come.
What are your thoughts on how developing countries can balance industrial development with public health needs in today’s global economy?
Academic Abstract:
This paper examines the evolution and transformation of India’s pharmaceutical industry in the context of economic reforms, WTO commitments, and changing patent regulations. Through analysis of historical policy frameworks, industry data, and emerging trends, it investigates how India successfully developed from an import-dependent nation to a global leader in pharmaceutical production and exports. The study demonstrates that an integrated policy approach combining process patent protection, price controls, and support for domestic R&D enabled India to build robust indigenous capabilities in pharmaceutical manufacturing. However, the study findings indicate that recent policy shifts, including TRIPS compliance requirements and economic liberalization, pose significant challenges. Analysis of empirical data suggests potential drug price increases of 26-242% following product patent implementation, while domestic R&D intensity remains relatively low at 1.55% compared to global standards. The research also evaluates strategic policy options for sustaining industry competitiveness while maintaining affordable drug access, including strengthened R&D investment, exploitation of traditional medicine opportunities, and strategic use of TRIPS flexibilities. It concludes that while India’s pharmaceutical sector faces substantial transitions, carefully crafted policies balancing innovation and access could help preserve its dual role as a competitive industry and provider of affordable medicines. The study contributes to broader discussions on industrial policy, intellectual property rights, and public health in developing economies.
Learn More:
Full citation: N. Kumar and Pradhan, Jaya Prakash (2003), ‘Economic Reforms, WTO and Indian Drugs and Pharmaceuticals Industry: Implications of Emerging Trends’, CMDR Monograph Series, No.-42, Dharwad: Centre for Multidisciplinary Development Research.
Learn More: